A lot has happened in the ten years since we embraced what we termed “Reality Labeling” on GOLDEN Acrylics, as reported in Issue 8 of Just Paint, published in April of 2001. At that time, we announced our decision to incorporate advice on our labels indicating to the user that “most chemicals are not fully tested for chronic toxicity”, and advised that the term “absence of known hazards” not be taken as a guarantee that a product is “non-toxic”. At the same time, we explained the introduction of “California Proposition 65 Warnings” on our labels, which alert the user to the presence of certain chemicals that are “Known to the State of California to cancer, birth defects, or other reproductive harm”. As we expected, our experience is that this phrase is too often interpreted as meaning the products themselves are known to cause such health problems.
As far as California goes, the list of products affected has increased. Carbon Black pigment was added to the state’s list and at this writing it was announced that the state intends to add Titanium Dioxide (the pigment in Titanium White) to the list. Both of these pigments are listed with the qualification that the warning applies to “airborne particles of respirable size1“. Both pigments are in our Airbrush, Fluid and Heavy Body lines. Usually, the Airbrush; often, the Fluid; and occasionally, the Heavy Body colors are spray-applied, and a fraction of the spray particulate may be of respirable size, so we will obligingly include the warnings on the labels of affected products. We will brace ourselves for the calls and emails from concerned users, and reiterate the toxicological improbability of harm, even more so if one takes reasonable precautions not to inhale spray mists.
Californians, in our experience, are usually accustomed, and often even apathetic or sardonic in regard to such labeling. However, as a manufacturer, we bear the onus to reconcile Prop 65 warnings with the labeling requirements of not only United States federal regulations, but those of other nations as well. It’s not just a question of making the logistics of conflicting requirements work; we want labeling practices to make sense. Shouldn’t there be one consistent label statement regarding health effects, regardless of where in the world the product is destined? And shouldn’t there be some mechanism to ensure that this statement is as complete and correct as possible? Change for the better, in regard to these issues, has emerged over the past decade in the form of Europe’s REACH initiative and the development of the Global Harmonized System (GHS) for preparing Safety Data Sheets and labeling products for health hazards.
REACH, standing for the Registration, Evaluation, Authorization and restriction, of Chemical substances, is Europe’s laudable effort toward correcting long-standing deficiencies in society’s manner of introducing chemical substances and their subsequent preparations into commerce. As we have seen repeatedly, chemical-based products are often on the market for significant periods of time, only to have it become known that they carry serious health risks. This is attributable to a strong economic incentive to innovate, less than stellar regulatory oversight, and arguably, an imperfect body of methodology to identify chronic health hazards.
To their credit, the European Parliament is leading the world in attempting to overcome these deficiencies through the complex set of regulations promulgated under REACH. These require that manufacturers and importers register the amount of each chemical substance put on the market in the European Community; and require that those responsible for the sale of the substances, either in pure form or as part of another product, assemble a thoroughly defined panel of toxicological and environmental effect data. At the same time, the regulations require that data is shared among all those involved, in order to expedite the process, ensure completeness, and eliminate duplicate animal testing. The data collected is evaluated by the authorities and chemicals are then authorized for use if they do not pose an unreasonable risk to health or the environment. If they do, the chemicals may be authorized only for uses deemed to have a greater societal benefit than risk; or chemicals will be banned altogether. We are still in the Evaluation stage of this process. By the time it is complete, the need to state that “not all chemicals are fully tested for chronic toxicity” will be greatly diminished.
The Global Harmonized System is an effort led by the United Nations to unify the content and format of Safety Data Sheets, and the warning phrases and symbols used on product labels, amongst countries. To that end, guidelines for these practices were developed and await adoption by the agencies responsible for occupational health and safety, transportation, and consumer labeling in participating countries.

Again, Europe has led the way in adopting GHS for product labeling. New requirements for pure substances were put in place in 2010, and will go into effect for preparations (mixtures) in 2015. In the US, OSHA (The Occupational Safety and Health Administration) has begun the process for incorporating GHS into the Hazard Communication Standard, but CPSC (The Consumer Product Safety Commission) has to date, shown little progress in updating its consumer product labeling regulations. Canada, however, is making progress toward doing so.
As a relative niche product in a global market, we are always trying to reconcile conflicting labeling requirements, and we see both these initiatives as positive, albeit painstakingly slow, progress toward a world-wide unified approach to toxicological review and appropriate labeling of products.
As mentioned, an outcome of the GHS will be the unification of hazard symbols on consumer labels. Historically, Europe has used the St. Andrews Cross, the United States uses no symbols for sub-lethal acute and chronic toxicity, and Canada has its own set of pictograms for acute hazards, but none for chronic. As described in our article in this publication 10 years ago, we chose to embrace the St. Andrews Cross for our world-wide labels, as we felt it was important to quickly alert consumers to the need to read the labels and follow directions for particular products, such as MSA Varnish, Acrylic Flow Release and Cadmium Colors. By 2015, we will discontinue the use of this symbol as it is not part of the GHS system and we will begin use of the international symbols.
Meanwhile, we have also been working to meet the requirements for internationally harmonized Safety Data Sheets (SDS). For the first time, it is required that the label warnings be reflected verbatim on SDS, and this has required that we make some interim changes on labels of some of our products that carry health warnings. Here, the challenge is that Europe makes it both easy and difficult in requiring that only prescribed wording may be used on labels. Since their Risk and Safety phrases are published in each of the 18 languages we include on our labels, it makes it easy to get the exact, correct phrases that are most appropriate and universally understood. However, understandably, not every usage scenario is envisioned, and in the past, we have customized some of these warnings to present the information that we deemed most relevant to the user of our products. Due to our immediate need to Harmonize in Europe, ultimate desire to do so world-wide; and the impeding demise of the St. Andrews Cross as the official European hazard symbol, some of our labels are currently in transition.
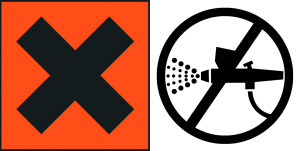
While we are not allowed to deviate from the official European risk and safety phrases when using the St. Andrews Cross, we are allowed to include “supplemental label information” as we deem appropriate. This, in combination with the theme of the increased use of pictograms on labels to break language barriers, has led to the following near-term changes of significance on GOLDEN labels:
Cadmium Colors: Use of the St. Andrews Cross is being discontinued and in its place, a pictogram of our own design is being used, indicating that the product should never be spray applied.
Acrylic Flow Release: We will continue to use the St. Andrews Cross for the present, and have added the pictogram indicating that the product should never be spray applied
GAC 900: Use of the St. Andrews Cross is being discontinued and in its place, we have added our pictogram indicating that the product should be used with ventilation because it releases small amounts of formaldehyde when heat-set. Without ventilation, a user could be exposed to a few parts per million in their work space. This amount isn’t chronically significant, but individuals sensitive to the compound could react to it.
Gel Topcoat: We have eliminated the St. Andrews Cross to align with European requirements, as the theoretical possibility of allergic reaction is limited to only those users who are already sensitized from a significant exposure to a constituent component of the product.
In addition to these changes, you may notice that the selection of some of our Risk and Safety Phrases on European labels for Acrylic Flow Release, MSA Varnish and Archival Varnish have been slightly modified based on recent review, reconciliation with our European SDS and expansion into many more EU markets. This effort has also necessitated that we eliminate Directions for Use of some of these products from the product label, in favor of presenting only Risk and Safety phrases in the multiple languages required. The Directions for Use are now included separately, where necessary, also in a multitude of languages to serve our world-wide markets.
The ongoing work of maintaining product labels presents an opportunity to both learn and educate, as well as refine our approach, as we transition to Global Harmonization. Above all, our goal continues to be to present the user with the information needed to make informed decisions about how to safely use our products.
1Resirable size particles are those less than 10 microns in diameter. Airbrushes can deliver mists well below this level. Pigment particles are often less than 1 micron in diameter.
About Ben Gavett
View all posts by Ben Gavett -->Subscribe
Subscribe to the newsletter today!
No related Post
