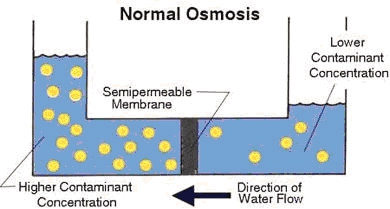
A semi-permeable membrane, like the membrane of a cell wall or a bladder, is selective about what it allows to pass through, and what it prevents from passing. These membranes in general pass water very easily because of its small molecular size; but also prevent many other contaminants from passing by trapping them. Water will typically be present on both sides of the membrane, with each side having a different concentration of dissolved minerals. Since the water in the less concentrated solution seeks to dilute the more concentrated solution, water will pass through the membrane from the lower concentration side to the greater concentration side. Eventually, osmotic pressure (seen in the diagram below as the pressure created by the difference in water levels) will counter the diffusion process exactly, and equilibrium forms.
The process of reverse osmosis forces water with a greater concentration of contaminants (the source water) into a tank containing water with an extremely low concentration of contaminants (the processed water). High water pressure on the source side is used to “reverse” the natural osmotic process, with the semi-permeable membrane still permitting the passage of water while rejecting most of the other contaminants. The specific process through which this occurs is called ion exclusion, in which a concentration of ions at the membrane surface form a barrier that allows other water molecules to pass through while excluding other substances.
Copyright 2007 by the Water Quality Association – Reprinted with Permission
About Golden Artist Colors, Inc.
View all posts by Golden Artist Colors, Inc. -->Subscribe
Subscribe to the newsletter today!
No related Post