By Vaikunt Raghavan, Ph.D.
Basics of Wetting and Adhesion
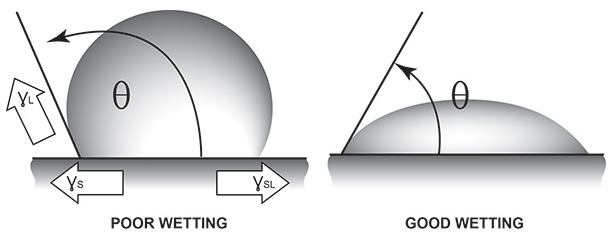
When a liquid comes into contact with any solid, new interfaces or boundaries are generated between these dissimilar materials. Although many factors exist which will promote or inhibit adhesion of the acrylic paint onto the solid plastic, the most important element is the ability of the liquid to ‘wet-out’ the solid onto which it is painted. When we see a drop of water bead up on plastic, we are seeing the dynamic of surface tension played out. What is a bit confusing is that, not only does the liquid have its own surface tension (liquid/air interfacial tension), but the solid onto which it is placed also has its own surface tension (solid/air interfacial tension or ‘surface energy’ in solids) as well. In fact, it is the surface tension of the solid (ɣs) which attempts to spread the liquid across the surface. Two forces work to oppose this spread. One is the surface tension of the liquid (ɣL). The higher the surface tension of the liquid, the greater the liquid’s attraction to itself. This attempts to reduce the surface area of the liquid. The other opposing force is called the interfacial tension (solid/liquid – ɣsL), which attempts to minimize the area of contact between the solid and liquid.1
When the contact angle (θ) is small (less than 90°), the liquid will tend to spread across the surface of the solid indicating good wetting. This happens when the surface tension of the liquid is low compared to the solid.
When the contact angle (θ) is zero, the liquid will tend to spread spontaneously across the surface of the solid indicating perfect wetting.
When the contact angle (θ) is large (greater than 90°), the liquid will tend to bead upon the solid indicating poor wetting. This happens when the surface tension of the liquid is high compared to the solid. Simply stated, for adequate wetting out of a liquid onto any solid, the surface tension of the solid must be higher than that of the liquid.
A coating must not only adequately and uniformly wet out the surface, but also adhere well to that surface. Therefore, in addition to spreading wetting it should also have good “adhesional wetting (WA)” which is defined mathematically as
WA = ɣs + ɣL – ɣsL
This equation tells us that in order to maximize adhesional wetting (WA) one must keep interfacial surface tension ɣsL as low as possible.
If I’ve lost you at this point, don’t worry, but the fact is that artists are constantly having to deal with the issues of one surface wetting out another to create good contact and subsequently good adhesion of their materials. This is especially evident when artists are using materials that are quite dissimilar in nature. Most of us have seen examples of our paints pooling up on the surface or creating little rivers on top of what we thought would be a continuous film. Or how many of you have used our Acrylic Flow Release or some other flow additive to get your paints to lay down or penetrate better into a surface? What you’ve experienced is simply the tension between the forces listed above. Sort of makes you wish you paid more attention in science class, doesn’t it?
Adhesion to Plastics
Surface tension actually has its own unit of measurement called dynes or dynes per centimeter, expressed dynes/cm. Clean, well prepared metal surfaces have high surface energies in the order of 400 to 1800 dynes/cm (Aluminum ~500, Copper ~ 1300, Nickel ~ 1800). They are wetted easily by acrylic polymers that have relatively low surface tension in the order of 40 to 50 dynes/cm. Wetting of plastic surfaces, on the other hand, is much more complex than wetting metal surfaces. Plastics being non-porous, non-polar or low-polar, hydrophobic, and being low surface energy materials are difficult to adhere to not just for artists, but also within the automotive, electronics and medical industries.
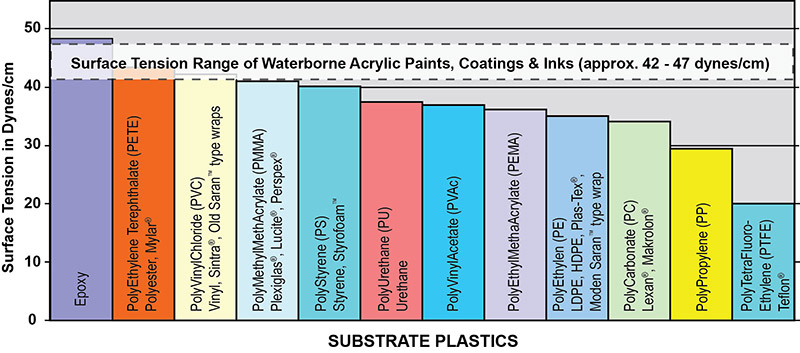
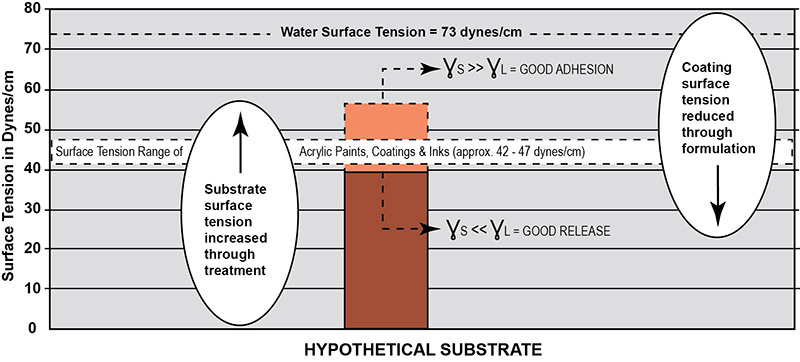
Plastic surfaces and paints are both polymeric materials and thus have similar surface tensions. There is considerable surface tension closeness or overlap within the narrow band between the plastic substrates (20 to 50 dynes/cm) and the waterborne acrylic dispersion polymers (39 to 47 dynes/cm) as shown in Figure 2. Adhesion between paint and the substrate is dependent on many factors such as differential surface tension, differential expansion to hot and cold, differential modulus (a measure of stiffness), shrinkage during drying/cure, the effects of solvents in the formulation, chemical structure, etc. Yet the most important factor determining adequate adhesion is the differential surface tension. The absence of a large difference between the critical surface tension of the paint and plastic substrates often results in poor surface adhesion or even complete failure. As a general rule, acceptable bonding adhesion is achieved when the surface energy of a substrate (measured in dynes/cm) is approximately 10 dynes/cm greater than the surface tension of the liquid. In this situation, the liquid is said to “wet out” or adhere to the surface. For this to happen, either the surface tension of the substrate or the paint must break loose of the logjam in Figure 2. An illustration of the surface energy of a hypothetical plastic substrate for good adhesion to waterborne acrylic is presented in Figure 3. As shown here, the higher the surface energy of the solid substrate relative to the surface tension of a liquid, the better its wettability will be, and the smaller the contact angle.
Adhesion strength is generally determined by the properties of a base material and its interface. Optimizing adhesion strength can be accomplished by modifying these interfaces physically and chemically. In formulating waterborne acrylic paints, the use of surface acting modifiers (surfactants) is essential in driving down the surface tension of the liquid paints. These materials can take the acrylic dispersion polymer’s surface tension down between 26 to 31 dynes, allowing wetting out of many difficult to coat surfaces.
Improving Coating Compatibility
Cleaners, Degreasers
Surface contamination is the most common reason for premature coating failures. There are many cleaners and degreasers available that are specifically designed to remove dust, dirt, loose particles, trace oils, waxes, grease, silicones, films, oxides, additives, plasticizers, mold release agents and even fingerprints! They range from low VOC products to 100% solvents. Some plastics such as flexible vinyls have very slow evaporating plasticizers that make coating with acrylic paints a problem as they can release those plasticizers to the surface leaving a sticky surface mess.
Primers, Tie-coats & Additives
Primers typically consist of a reactive chemical or binder mixed in a solvent. It is either brushed or sprayed onto the substrate surface. The solvent evaporates, leaving the active material behind. Depending on the type of primer, the surface may be ready to bond immediately or may require time to dry thoroughly. Primers are commonly used on PET (Mylar®), Polyurethanes and silicones. Tie-coats typically act as a chemical bridge between the plastic and the paint. They may simply be an adhesion promoting agent in low concentration dispersed in solvent/water. It is usually applied as a very thin coat. The application of a tie-coat directly to the plastic’s surface may eliminate the need for a scuffing process which often results in visible scratch marks.
Silanes are a popular class of adhesion promoters used if the plastic has appropriate functional groups. Special classes of additives known as chlorinated polyolefins (CPOs) are also used as adhesion promoters for paints, coatings and inks on plastics such as untreated polyethylene, polypropylene or thermoplastic polyolefins (TPOs).
These CPOs are used in at least three ways:
- As a primer between the substrate and subsequent coatings
- As a tie-coat between coating layers
- As an additive to the paint
Improving Substrate Compatibility
Plastic’s surface preparations and/or modifications can greatly improve the acceptance of paints. Bond strengths and functional performance can be dramatically improved by increasing the water-loving (hydrophilic) and surface energy characteristics to promote adhesion, thereby adding value to the product and the process.
Surface Roughening
This is a simple and easy method for artists and quite effective for many plastics. Though it does not change the surface tension of the plastic substrate, the improved bondability comes from increasing the number of mechanical interlocking sites. Abrading a surface gets rid of films, scales and oxides, increasing the area available for bonding. It is important before abrading to first degrease the surface, as it might be more difficult to remove wax or oils from the spaces within the etched surface. After abrading with fine grain sandpaper or emery cloth, make sure to remove all loose particles with a brush or compressed air and then degrease again to remove all residual oils and mold release agents.
The sanded or etched surface will provide opportunities for the acrylic coating to find many small areas to attach to. This can be dramatically influenced by the viscosity (thick to thin) and rheology (flow properties) of the paint. Too thick a paint will not penetrate into the tiny recesses of the etched surface. Paints with poor flow properties won’t easily move into and around the bumpier profile of the sanded surface. As a general rule, thinner products will typically provide better penetration and therefore, better adhesion to the plastic surfaces.
Chemical Bonding
There is only limited chemical bonding between the typical plastic and the liquid acrylic paint. A plastic resin is considered polar if its charge can be measured as positive or negative. If a polymer has no charge, we say it is non-polar. Many plastics are non-polar, so the opportunity for adhesion is really limited. Unless polyethylene or polypropylene are specially treated (such as flame treatment), their surface energy is so low as to severely limit adhesion. Polyethylene sheeting becomes a great surface for making acrylic skins that can easily be lifted off after drying because of the poor adhesion.
Acrylic Plastics (PMMA), ABS plastics and Polycarbonates are examples of polar plastics and therefore, typically have higher surface energy and improved adhesion for acrylic paints.
Other Treatments for Improving Adhesion of Coating to Plastics
There are a range of treatments used to improve the adhesion of paints to a range of plastics. These require industrial methods to process these substrates. The treatments include Flame Treatment2, Thermal Treatment3, Corona Discharge4, Plasma Treatment5, Chromic Acid Etching6, and Adhesive Abrading7. There are several other treatments such as iodine and sodium and techniques such as surface grafting, transcrystalline growth, gamma radiation and UV exposure that have been successfully employed to increase surface energy of plastics that find mention in the literature. These are mostly used on difficult to wet substrates such as polyamides, fluoropolymers and polyolefins in industrial settings.
Recommendations for Artists
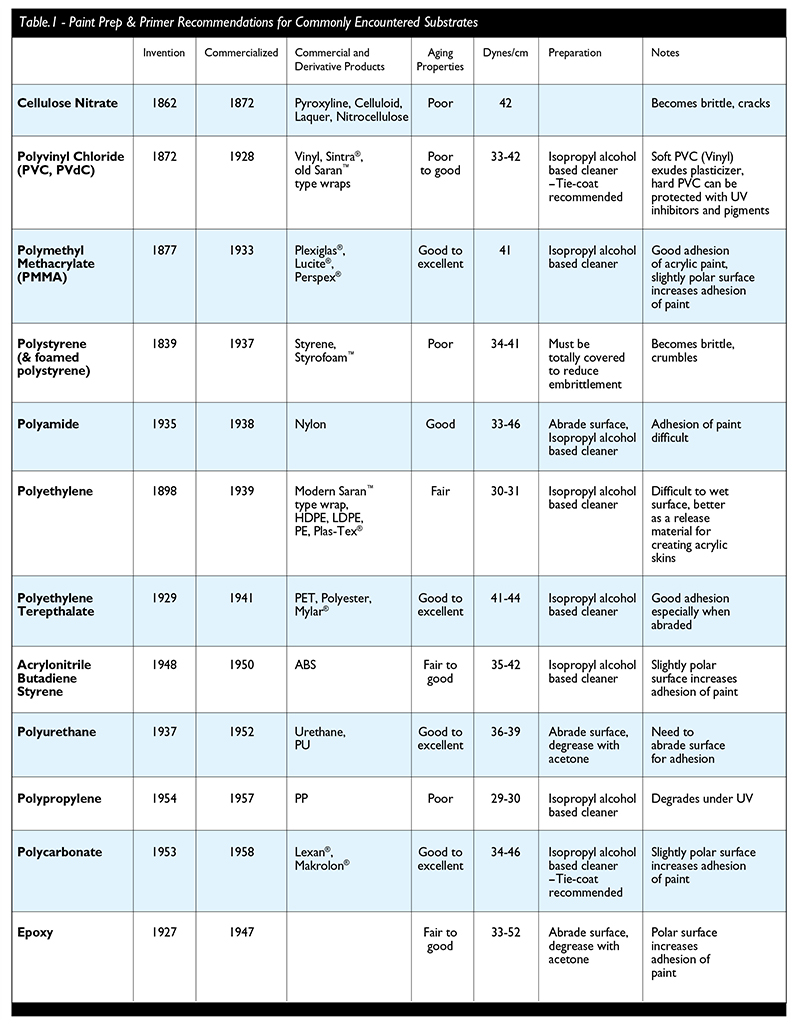
Some preliminary recommendations are presented in Table 1 for achieving improved adhesion between the acrylic paint and the plastic substrate (by no means are these all inclusive). The rigid, lightweight, smooth surfaces of modern plastics provide a very tempting substrate for artists to use in their work. As is their right, artists can use and will use all these materials in their various forms for constructions and painting surfaces. It is possible to choose amongst the various plastics to use those that are most likely to produce durable results. Those would certainly include the acrylic, polycarbonate, polyester (PET), urethane plastics and some of the hard PVC Plastics. Other plastics in common use for artists include a range of vinyls and surfaces made with polypropylene plastic. Technologies are constantly being advanced to create new plastic combinations and surface treatments for improved compatibility with coatings. These new materials will allow artists to explore an increasing array of working possibilities. Yet it is clear that not all plastics are created equal and although they all may be tempting, make sure you’re choosing the material for the right reasons.
Sorting Through the Plastics
Tempting as these flat, light, semi-rigid, transluscent or transparent materials seem to be as painting supports, the use of them by artists should have a “use at your own risk” warning sign. This article is not an attempt to define all of these possible supports, but to give what we think is a valuable brief on the concepts of adhesion of the acrylic paints and mediums onto these supports. The discussion of the various characteristics of each of these plastic supports will have to wait for another article.
Foot Notes
1. The balance of these forces in figure 1 may be mathematically expressed as: ɣs = ɣsL + ɣL (cos θ). The contact angle (θ) is the angle at which the liquid contacts the solid (Figure 1). This contact angle is a measure of “spreading wetting” of a surface.
2. Flame Treatment: This is a process of oxidizing the surface of the substrate by briefly exposing it to flame to introduce functional groups. This increases surface energy and improves bonding. The adiabatic flame temperature is around 1800° C. Flame treated surfaces often retain more stable aging than corona treated surfaces. Flame treatment is used in treating PE, PP and PET.
3. Thermal Treatment: Thermal treatment is very similar to flame treatment except that it exposes the substrate to a blast of hot air around ~500° C instead of flame to oxidize the surface. It also involves a free radical mechanism accompanied by some surface cross-linking and chain scissions which improves wettability and adhesion.
4. Corona Discharge: A process of roughening the surface of the substrate by high voltage across a gap to introduce reactive sites and facilitate mechanical interlocking through a corona discharge at atmospheric pressure in the presence of air. The effectiveness is not permanent so it is best to paint immediately after treatment. It works well for polyethylene, polypropylene and polyester.
5. Plasma Treatment: This is an electrical process that uses ionized air to increase the surface tension of non-porous, non-polar plastic substrates, thereby improving the wettability and adhesion of paints, coatings, adhesives and inks. In air plasma discharge electrons bombard the surface with high energies that break the molecular bonds on the surface. The resulting free radicals rapidly react on the molecular chains on the surface resulting in some cross-linking. As a result of polar groups created on the surface, oxidative effects increases surface energy. Humidity and temperature degrade the treatment rapidly and it is imperative that the surface be coated within a short period of time, usually minutes. There are easy to operate, portable, hand-held plasma treaters, which generate gentler and lower temperature plasma ideal for delicate surfaces, available in the market.
6. Chromic Acid Etching: A process that introduces to the surface of the substrate reactive functional sites such as carboxylic acid/hydroxyl/carbonyl groups and chemical etching to facilitate mechanical interlocking and improve bonding characteristics. It is recommended for polypropylene and polystyrene.
7. Adhesive Abrading: This is a process of abrading the surface of the substrate, especially Teflon®, in the presence of an adhesive to create free radicals that react with the adhesive itself.
8. Wettability, Spreading, and Interfacial Phenomena in High Temperature Coatings, JOM-e, 52(1) (2000), a publication of The Minerals, Metals & Materials Society
References
SpecialChem4Adhesives-Adhesion Guide http://www.specialchem4adhesives.com
The Loctite Design Guide for Bonding Plastics, Vol. 6 http://www.henkelna.com
Plastic Surface Modification – Surface Treatment, Decoration, and Adhesion, Rory A. Wolf, Hanser Publishers, 2010.
Formulating with Eastman waterborne CPO adhesion promoters http://www.eastman.com
Adhesion Bonding-Industry Resources & Connections http://www.adhesionbonding.com
Enercon – Plasma surface treating systems http://www.enerconind.com
Matthews Paint Substrate Preparation Guide http://www.matthewspaint.com
Surface Prep Guide for Adhesives http://www.adhesive.com
Trends in Plastic Coatings, Coating Plastics by Lawrence C. Van Iseghem, Van Technologies, Inc.
Protective Coatings by Clive H. Hare, Technology Publishing Company, Pittsburgh, PA
Surfactants and Interfacial Phenomenon by M.J. Rosen, John Wiley & Sons
Accu Dyne Test™ – Tables of Polymer Surface Characteristics http://www.accudynetest.com
About Vaikunt Raghavan
View all posts by Vaikunt Raghavan -->Subscribe
Subscribe to the newsletter today!
No related Post

Nice article.Very informative .As we know that paints are used to protect the things from corrosion,heat ,temperature.Before painting the plastics we need to make the surface clean of the plastic so that the bonding between the plastic and the element is stronger.Great article .Thanks for posting.
Very helpful article, many thanks
Hi Jim –
Thanks! We truly appreciate the warm feedback and if there is anything else we can ever do, just ask!
In my experience, rubbing alcohol can produce cracks on acrylic, yet it is specified in Figure 4 as a prep cleaner. Can you explain?
Amazing article!